Hello.
First, let's recall the Properties of Exponents:
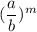 =
=
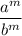
Let's use this property to simplify the given expression:
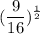
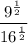
Now, the
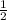 power means "square root of"
power means "square root of"
The square root of 9 is 3, and the square root of 16 is 4.
Thus, we have
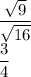
Therefore, the answer is

I hope it helps.
Have a nice day.