Answer:
The mass of Element X will reach 62 grams in 7.0 years.
Explanation:
We can find the time of decay of Element X by using the exponential decay equation:
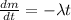
The solution of the above equation is:
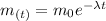 (1)
(1)
Where:
t: is the time =?
λ: is the decay constant
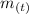 : is the mass at time t = 62 grams
: is the mass at time t = 62 grams
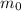 : is the initial mass = 90 grams
: is the initial mass = 90 grams
First, we need to calculate λ
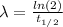 (2)
(2)
Where
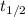 is the half-life = 13 years
is the half-life = 13 years
By entering equation (2) into (1) and solving for "t" we have:
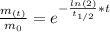
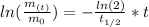
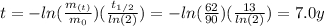
Therefore, the mass of Element X will reach 62 grams in 7.0 years.
I hope it helps you!