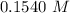Answer:
"0.1540 M" is the correct answer.
Step-by-step explanation:
The given values are:
Mass of sample,
M = 6.04 g
Volume,
V = 100 mL
or,
= 100×10⁻³ L
Molar weight of Chromium(III) sulfate
MW = 392.16 g/mol
Now,
The molarity will be:
=
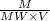
By putting the values, we get
=
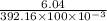
=
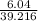
=