Answer:
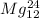 and
and
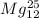
Step-by-step explanation:
Isotopes can be defined as the atom of an element that has the same number of protons but different number of neutrons. This ultimately implies that, the isotopes of an element have the same atomic number (number of protons) but different atomic mass (number of nucleons).
The isotope of an element is denoted by
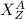
Where;
X is the symbol of the element.
A is the atomic mass or number of nucleons.
Z is the atomic number or number of protons.
Mathematically, the number of neutrons = A - Z
In this scenario, the two atoms which are isotopes of each other are
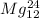 and
and
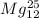 because they have the same atomic number (number of protons) but different atomic mass (number of nucleons).
because they have the same atomic number (number of protons) but different atomic mass (number of nucleons).