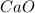Answer:
Calcium atom has two valency electrons in its outermost energy level and these participate in bonding with the two of the six valency electrons in the outermost energy level of oxygen atom.
Calcium loses these two electrons and it gains a charge of +2 while Oxygen atom gains these two electrons and gains a charge of -2 hence gains stability
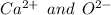
As a result, Calcium oxide is formed due to ionic or electro valent bonding.