1.
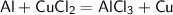

2. CuCl₂·2H₂O is limiting reactant.
Chemical reaction: 3CuCl₂·2H₂O + 2Al → 3Cu + 2AlCl₃ + 6H₂O. m(Al) = 0,5 g.

3. The molar mass of copper is 63.546 grams per mole.
If we multiply everything out, we'll get 0.50722 grams of copper, which is our theoretical yield.


Hope it helps uh :)♡~