Answer:
Step-by-step explanation:
We are finding the pressure with a change in temperature, so we should use Gay Lussac's Law. This states that the pressure is directly proportional to the temperature. The formula is:
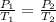
The original pressure is 1.74 atmospheres and a temperature of 273 Kelvins. When the gas is heated the new temperature is 312 Kelvins, but the new pressure is unknown. Substitute all the known values into the formula.
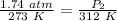
Since we are solving for the new pressure, we need to isolate the variable P₂. It is being divided by 312 Kelvin and the inverse of division is multiplication. Multiply both sides by 312 K.
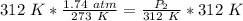
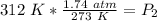
The units of Kelvin (K) will cancel.
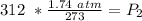
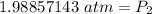
The original measurements have 3 significant figures, so our answer must have the same. For the number we found, that is the hundredth place.
The 8 in the thousandth place tells us to round 8 in the hundredth place up to a 9.
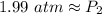
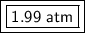
The new pressure is approximately 1.99 atmospheres.