Answer:
The original solution’s concentration was 3.09 M.
Step-by-step explanation:
Dilution is the reduction of the concentration of a chemical substance in a solution, and consists simply of adding solvent to an existing solution.
The quantity or mass of the solute is not changed but only that of the solvent. That is, as only solvent is being added, the consequence is that by not increasing the amount of solute but if the amount of solvent, the concentration of the solute decreases.
A dilution is expressed as:
Cinitial*Vinitial = Cfinal*Vfinal
In this case:
- Cinitial= ?
- Vinitial= 8.4 L
- Cfinal= 0.65 M
- Vfinal= 40 L
Replacing:
Cinitial*8.4 L = 0.65 M*40 L
Solving:
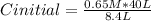
Cinitial= 3.09 M
The original solution’s concentration was 3.09 M.