Answer:
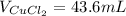
Step-by-step explanation:
Hello there!
In this case, according to this titration problem, it is possible to note there is a 1:3 mole ratio of CuFeS2 to CuCl2; it means that we can use the following equation:
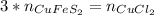
Thus, by introducing the mass and molar mass of the former and the volume and concentration of the latter, we write:
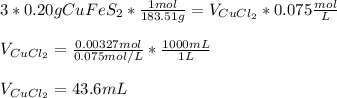
Regards!