Answer:
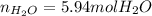
Step-by-step explanation:
Hello there!
In this case, according to the given question about stoichiometry, it is possible for us to calculate the required moles of water that will be produced by 12 grams of hydrogen, by using the molar mass of this reactant (2.02 g/mol as it is diatomic) and the 2:2 mole ratio in the chemical equation by solving the following setup:
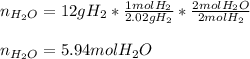
Regards!