Answer:
5.25 moles.
Step-by-step explanation:
The decomposition reaction of NaN₃ is as follows :
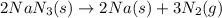
We need to find how many grams of N₂ produced in the process.
From the above balanced chemical reaction, we conclude that the ratio of moles of sodium azide and nitrogen gas are 2 : 3.
2 moles of sodium azide decomposes to give 3 moles of nitrogen gas. So,
3.5 moles of sodium azide decomposes to give
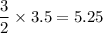 moles of nitrogen gas.
moles of nitrogen gas.
Hence, the number of moles produced is 5.25 moles.