Answer:
Q = 5254.39 J
Step-by-step explanation:
Given that,
Mass of ethanol, m = 68.8 g
The temperature increases from 40.0°C to 71.3°C.
The specific heat of ethanol is 2.44 j/g°C
We need to find the heat absorbed by the ethanol. We know that the heat absorbed due to the change in temperature is given by :
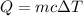
Put all the values,
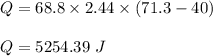
So, 5254.39 J of heat is absorbed by Ethanol.