The complete question is: Is the compound
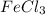 ionic or covalent ?
ionic or covalent ?
Answer: Compound
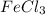 is an ionic in nature.
is an ionic in nature.
Step-by-step explanation:
When a metal atom combines with a non-metal atom then transfer of electrons take place from metal towards the non-metal. As a result, an ionic bond is formed between the atom and compound formed is called an ionic compound.
For example,
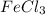 compound contains Fe atom which is a metal and Cl atom which is a non-metal. Hence, electrons will be transferred from Fe atom to the Cl atom forming an ionic bond.
compound contains Fe atom which is a metal and Cl atom which is a non-metal. Hence, electrons will be transferred from Fe atom to the Cl atom forming an ionic bond.
Hence,
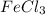 is an ionic compound.
is an ionic compound.
Thus, we can conclude that
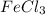 is an ionic compound.
is an ionic compound.