Answer:
P = 1.26 atm
Step-by-step explanation:
Given that,
The number of moles, n = 1.42 mol
Volume, V = 28.1 L
Temperature, T = 305 K
We need to find the pressure of the gas. The ideal gas law is as follows :
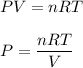
R = 0.0821 L-atm/mol-K
Put all the values,
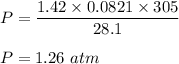
So, the pressure of the gas is 1.26 atm.