Answer: The molar mass of the gas is 9.878 g/mol.
Step-by-step explanation:
According to Graham's law, the rate of diffusion is inversely proportional to square root of molar mass of gas.
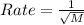
where,
M = molar mass of gas
As given gas diffuses 1/7 times faster than hydrogen gas. So, its molar mass is calculated as follows.
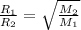
where,
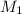 = molar mass of hydrogen gas
= molar mass of hydrogen gas
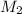 = molar mass of another given gas
= molar mass of another given gas
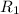 = rate of diffusion of hydrogen
= rate of diffusion of hydrogen
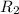 = rate of diffusion of another given gas =
= rate of diffusion of another given gas =
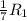
Substitute the values into above formula as follows.
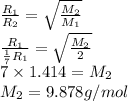
Thus, we can conclude that the molar mass of the gas is 9.878 g/mol.