Solution :
According to Henry's law of solubility, we have c = kp
Henry's law constant,
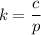
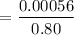
= 0.0007 mol/L.atm
When the pressure is = 4 atm
The solubility is c = 0.0007 mol/(L.atm) x 4 atm
= 0.0028 mol/L
Therefore, in a 5 liter of blood, the moles of nitrogen dissolved
= 0.0028 x 5
= 0.014 moles
At the surface, the solubility is = 0.00056 mol/L
So the moles of the nitrogen dissolved = 5 x 0.00056
= 0.0028 moles
Therefore, the number of moles of nitrogen released = 0.014 - 0.0028
= 0.0112 moles
Given total pressure = 1 atm
Temperature = 37 degree C
= 37 + 273
= 310 K
R =
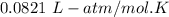
Therefore, volume of the nitrogen is
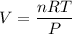
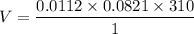
= 0.285 L