Answer:
1.6896 grams
Step-by-step explanation:
Given that:
Total pressure = The pressure of
 + partial pressure of
+ partial pressure of
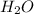
∴
The pressure of
 = Total pressure - partial pressure of
= Total pressure - partial pressure of
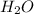
= (754.8 - 17.55) mmHg
= 737.25 mmHg
At standard conditions:
1 mmHg = 0.00131579 atm
∴
737.25 mmHg will be: (737.25*0.00131579) atm = 0.970065928 atm
P ≅ 0.97 atm
The temperature (T) = 293 K
Volume (V) = 1.31 L
Using ideal gas equation
PV = nRT
0.97 × 1.31 = n × 0.0821 × 293
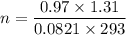
n = 0.0528
mass of oxygen
 = no. of moles * molar mass
= no. of moles * molar mass
= 0.0528 * 32
= 1.6896 grams