Answer: It will take 425 hours to plate 19.5 kg of copper onto the cathode if the current passed through the cell is held constant at 38.5 A.
Step-by-step explanation:
The half-reaction equation is as follows.
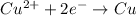
According to the standard values, 1 mole of an electron carries 96500 C. Therefore, charge carried by 2 moles of electrons is
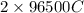 .
.
Also, atomic mass of Cu is 63.5 g. According to the equation 2 moles of electrons are depositing 63.5 g of Cu.
Hence, charge required to deposit 19.5 kg (1 kg = 1000 g) or 19500 g of Cu is calculated as follows.
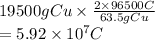
Formula used to calculate time is as follows.
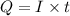
where,
Q = charge
I = current
t = time
Substitute the values into above formula as follows.
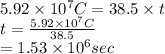
As 1 hour contains 3600 seconds. So, converting seconds into hours as follows.
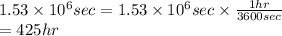
Thus, we can conclude that it will take 425 hours to plate 19.5 kg of copper onto the cathode if the current passed through the cell is held constant at 38.5 A.