Answer:
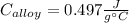
Step-by-step explanation:
Hello there!
In this case, according to this calorimetry problem on equilibrium temperature, it is possible for us to infer that the heat released by the metal allow is absorbed by the water for us to write:
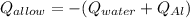
Thus, by writing the aforementioned in terms of mass, specific heat and temperature, we have:
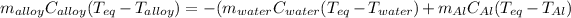
Then, we solve for specific heat of the metallic alloy to obtain:
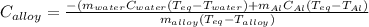
Thereby, we plug in the given data to obtain:
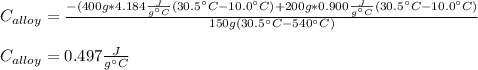
Regards!