Answer: 10. The reactant in Reaction B is
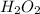 .
.
11. The products in Reaction B are
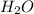 and
and
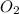 .
.
12. There are 2 molecules present on the reactant side of the equation.
13. There are 3 molecules present on the product side of the equation.
Step-by-step explanation:
The given reaction equation is as follows.
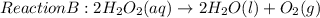
Reactants are the species present on the left side of a chemical equation.
Products are the species present on the right side of a chemical equation.
Therefore, the reactant in Reaction B is
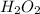 . As 2 is coefficient of
. As 2 is coefficient of
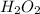 so, 2 molecules are present on the reactant side of the equation.
so, 2 molecules are present on the reactant side of the equation.
The the products in Reaction B are
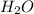 and
and
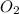 . The coefficient of
. The coefficient of
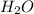 is 2 and coefficient of
is 2 and coefficient of
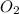 is 1. Hence, there are total (2 + 1) equals to 3 molecules present on the product side of the equation.
is 1. Hence, there are total (2 + 1) equals to 3 molecules present on the product side of the equation.
Thus, we can conclude that:
The reactant in Reaction B is
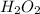 .
.
The products in Reaction B are
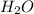 and
and
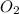 .
.
There are 2 molecules present on the reactant side of the equation.
There are 3 molecules present on the product side of the equation.