Answer: The specific heat capacity of the sample is
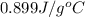 and as heat is released in this reaction so it is exothermic in nature.
and as heat is released in this reaction so it is exothermic in nature.
Step-by-step explanation:
Given: Mass = 120.4 g
Heat energy released = -7020 J
Initial temperature =
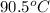
Final temperature =
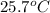
Formula used is as follows.
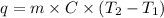
where,
q = heat energy
m = mass of substance
C = specific heat capacity
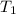 = initial temperature
= initial temperature
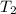 = final temperature
= final temperature
Substitute the values into above formula as follows.
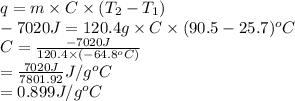
When heat is released in a process or reaction then it means it is exothermic in nature.
Thus, we can conclude that the specific heat capacity of the sample is
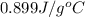 and as heat is released in this reaction so it is exothermic in nature.
and as heat is released in this reaction so it is exothermic in nature.