Given :
For a chemical reaction, the activation energy for the forward reaction is +181 kJ and the activation energy for the backward reaction is +62 kJ.
To Find :
The overall energy change for the forward reaction.
Solution :
The overall energy change for the forward reaction is :
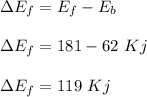
Therefore, the overall energy change for the forward reaction is 119 Kj.