Answer:
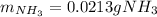
Step-by-step explanation:
Hello there!
In this case, since it is known that the reaction between ammonia and acetic acid is:
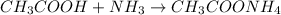
It is possible for us to realize that the mole ratio of acetic acid (vinegar) to ammonia is 1:1, that is why we can relate the concentrations as follows:
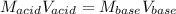
In such a way, by knowing that the volume of these two are the same, we infer that their concentrations is also de same; and therefore, the mass of ammonia is calculated as:
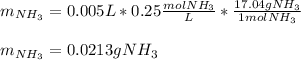
Regards!