Answer:
T₂ = 128.19 K
Step-by-step explanation:
Given that,
The initial volume, V₁ = 12.5 ft³
Initial temperature, T₁ = -173 °C = 100.15 K
Final volume, V₂ = 16 ft³
We need to find the new temperature. The relation between temperature and volume is given by :
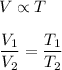
Put all the values,
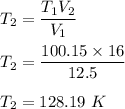
So, the new temperature is 128.19 K.