Answer:

Step-by-step explanation:
The equation for this problem is:
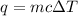
where m is the mass, c is the specific heat capacity, and ΔT is the change in temperature.
The mass is 3 kilograms, but the specific heat capacity includes grams in the units. Convert kilograms to grams. There are 1000 grams in 1 kilogram.
The specific heat capacity for lead is found on the table. It is 0.129 J/g°C.
Let's find the change in temperature. It is raised from 15 °C to 20 °C.
Now we know every value.
- m= 3000 g
- c= 0.129 J/g°C
- ΔT= 5 °C
Substitute the values into the formula.
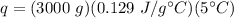
Multiply the first 2 numbers together. The units of grams cancel.
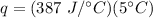
Multiply again. This time the units of degrees Celsius cancel.
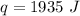
1935 Joules of energy are required and choice C is correct.