Answer: The given demonstration does not support Boyle's Law.
Step-by-step explanation:
According to Boyle's law, at constant temperature the pressure of an ideal gas is inversely proportional to the volume of gas.
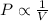
Therefore, an increase in volume of gas will lead to a decrease in pressure and vice-versa.
Now, according to the given data of volumes there is occurring an increase on moving from final volume 1 to final volume 3. This means that pressure is decreasing.
As a result, if a balloon was squeezed or compressed then it would get smaller. But in the given demonstration pressure is not given.
Thus, we can conclude that the given demonstration does not support Boyle's Law.