Answer:
43.1atm is the pressure using gas law and 27.2atm using Van der Waals Law.
Step-by-step explanation:
Ideal gas law is:
PV = nRT
Where P is pressure in atm
V is volume = 4.00L
n are moles of the gas (For chlorine Molar Mass: 70.90g/mol):
500g * (1mol / 70.90g) = 7.052 moles
R is gas constant = 0.082atmL/molK
T is absolute temperature = 25°C + 273 = 298K
To solve the pressure, P:
P = nRT/V
P = 7.052mol*0.082atmL/molK*298K / 4.00L
P = 43.1atm is the pressure using gas law.
Van der Waals equation is:
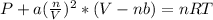
Where a is 6.58L²atm*mol⁻²
b = 0.056Lmol⁻²
Solving for pressure:
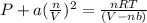
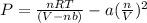
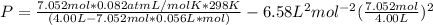
P = 172.323 / 3.6051 - 20.4866
P = 27.2atm using Van der Waals Law