Answer:
e. none of the above.
Step-by-step explanation:
Hello there!
In this case, according to the Rydberg's equation for the calculation of energy during electron transitions:
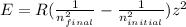
In such a way, we may assume that this element is H so z=1m the final energy level is 2 and the initial one is 3; thus, we plug in the values, and the Rydberg's constant to obtain:
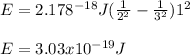
Thus, the answer is e. none of the above because the exponent is not given correctly.
Regards!