Answer:
Empirical: OH
Molecular:
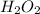
Step-by-step explanation:
First of all, we are going to use the formula: mass = no. moles x Molar Mass And rearrange it to find No. moles: No. moles = mass/Molar Mass
Let's start with Hydrogen:
The given mass is 0.44g, and hydrogen's molar mass is 1.01, therefore the number of moles is: 0.44/1.01 = 0.4356
Now we do the same for Oxygen:
Given mass = 6.92, Molar mass of Oxygen = 16.00, No. Moles = 6.92/16.00 = 0.4325
Now we identify the smaller one (Oxygen as 0.4325 < 0.4356) and we divide both values by that number:
0.4325/0.4325 = 1
0.4356/0.4325 = 1.01
We round both to the nearest 0.2 or 0.25 (depending on what you're taught), and we get: 1 and 1.
This means that the empirical formula has one of each: OH
Now to find the molecular formula we find the relative mass of OH and divide the given mass by that:
M(OH) = 16.00+1.01 = 17.01
34.00/17.01 = 2
We now multiply both by this number to get:
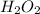
Hope this helped!