Answer: If the solubility of sodium chloride is 36 grams per 100 grams of water then 5.8 moles of NaCl dissolved in 1 L of water solution would be considered unsaturated.
Step-by-step explanation:
A solution which contains the maximum amount of solute is called a saturated solution. Whereas a solution in which more amount of solute is able to dissolve is called an unsaturated solution.
Now, the number of moles present in 36 g of NaCl (molar mass = 58.4 g/mol) is as follows.
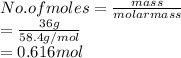
This shows that solubility of sodium chloride is 36 grams per 100 grams of water means a maximum of 0.616 mol of NaCl will dissolve in 100 mL of water.
So, a solution in which number of moles of NaCl are less than 0.616 mol per 100 mL then the solution formed will be an unsaturated solution.
- As 5.8 moles of NaCl dissolved in 1 L (or 1000 mL) of water. So, moles present in 100 mL are calculated as follows.
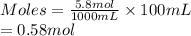
- Moles present in 100 mL of water for 3.25 moles of NaCl dissolved in 500 ml in water are as follows.
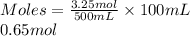
- Moles present in 100 mL of water for 1.85 moles of NaCl dissolved in 300 ml of water are as follows.
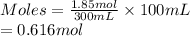
Thus, we can conclude that if the solubility of sodium chloride is 36 grams per 100 grams of water then 5.8 moles of NaCl dissolved in 1 L of water solution would be considered unsaturated.