Answer:
0.20 M
Step-by-step explanation:
Molarity is the concentration of a solute (MgCl2) in our solution (water). Molarity tells us how many moles of a substance is per Liter. So to figure out Molarity we'll break it down to this formula: Molarity =
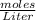 .
.
We can get moles by converting it from grams -> moles by using the molar mass, which is measured in grams/mol, that is our stepping stone to moles.
The molar mass is the sum of all the substances in the compound, so you'll need to refer to the periodic table.
Molar mass: (24g Mg) + ( 2 x 35g Cl)= 94g
Moles of MgCl2: 45.7g MgCl2 x
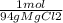 = 0.49 mol MgCl2
= 0.49 mol MgCl2
1 kg of water = 1 L so we don't have to convert anything, we can just use 2.40 L.
Molarity of MgCl2:
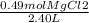 = 0.20M
= 0.20M