Answer:
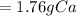
Step-by-step explanation:
Hello there!
In this case, according to the given atoms of calcium, it is possible to calculate the mass of this element by considering that the definition of mole is in terms of atoms and also the atomic mass of calcium:
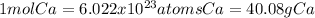
In such a way, by considering the following setup, we can obtain:
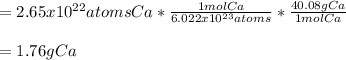
Best regards!