Answer:
The amount of heat transferred from the banana is (-)7.54 KJ
Step-by-step explanation:
As we know,

Q = Amount of heat transferred
m = mass of banana
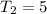 degree Celsius
degree Celsius
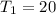 degree Celsius
degree Celsius
The amount of heat transferred from the banana =
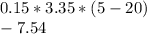 KJ (negative sign represents reduction in heat energy)
KJ (negative sign represents reduction in heat energy)