Answer:
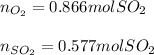
Step-by-step explanation:
Hello there!
In this case, according to the given balanced chemical reaction by which ZnS reacts with O2, it is possible to calculate the moles of the latter that are consumed, not produced, according the 2:3 mole ratio between them and the following stoichiometric set up:
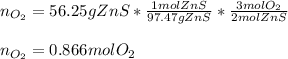
But also, we can compute the moles of SO2 that are produced via the the 2:2 mole ratio of ZnS to SO2:
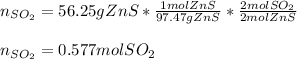
Regards!