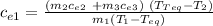Answer:
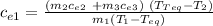
Step-by-step explanation:
This is a calorimeter problem where the heat released by the console is equal to the heat absorbed by the cupcake and the plate.
Q_c = Q_{abs}
where the heat is given by the expression
Q = m c_e ΔT
m₁ c_{e1) (T₁-T_{eq}) = m₂ c_{e2} (T_{eq} -T₂) + m₃ c_{e3} (T_{eq}- T₁)
note that the temperature variations have been placed so that they have been positive
They ask us for the specific heat of the console