Answer:
The final temperature is equal to 240 K or -33.15°C
Step-by-step explanation:
Given that,
Initial temperature of the gas, T₁ = 47°C = 320 K
Initial pressure, P₁ = 140 kpa
Final pressure, P₂ = 105 kpa
We need to find the final temperature if the volume remains constant. The relation between temperature and pressure is given by :
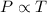
or
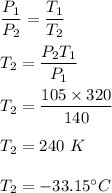
So, the final temperature is equal to 240 K or -33.15°C.