Answer:
b.
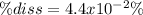
Step-by-step explanation:
Hello there!
In this case, given the ionization reaction of HClO as weak acid:
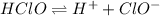
We can write the equilibrium expression as shown below:
![Ka=3.5x10^(-8)=([H^+][ClO^-])/([HClO])](https://img.qammunity.org/2022/formulas/chemistry/high-school/xkq1bk8jvchwdzlcjcyejxn8hila4neo4p.png)
In such a way, via the definition of x as the reaction extent, we can write:
![3.5x10^(-8)=(x^2)/([HClO])](https://img.qammunity.org/2022/formulas/chemistry/high-school/brjljrs0x9vmrsg38tkf751von47vntyu4.png)
As long as Ka<<<<1 so that the x on the bottom can be neglected. Thus, we solve for x as shown below:
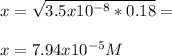
And finally the percent dissociation:
![\% diss=(x)/([HClO]) *100\%\\\\\% diss=(7.94x10^(-5)M)/(0.18)*100\% \\\\\% diss =0.044\%=4.4x10^(-2)\%](https://img.qammunity.org/2022/formulas/chemistry/high-school/eo4m2d9orsb2vx10pjtba5kjzsfr2e8gz5.png)
Which is choice b.
Best regards!