Answer:
10 moles of potassium Chlorate are required to produce 15 mole of oxygen
Step-by-step explanation:
The balanced chemical equation is
2KClO3(s)→2KCl(s)+3O2(g)
2 moles of potassium Chlorate produce 3 moles of oxygen
Number of moles of potassium Chlorate required to produce 1 mole of oxygen
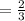
Number of moles of potassium Chlorate required to produce 15 mole of oxygen
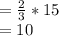
10 moles of potassium Chlorate are required to produce 15 mole of oxygen