Answer:
2.
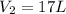
3.
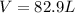
Step-by-step explanation:
Hello there!
2. In this case, we can evidence the problem by which volume and temperature are involved, so the Charles' law is applied to:
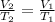
Thus, considering the temperatures in kelvins and solving for the final volume, V2, we obtain:
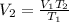
Therefore, we plug in the given data to obtain:
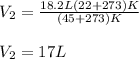
3. In this case, it is possible to realize that the 3.7 moles of neon gas are at 273 K and 1 atm according to the STP conditions; in such a way, considering the ideal gas law (PV=nRT), we can solve for the volume as shown below:
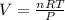
Therefore, we plug in the data to obtain:

Best regards!