Answer:
The volume of the flask is 20.245 litres .
Step-by-step explanation:
We are given with following information-
 -------- 1
-------- 1
where R =
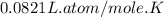
Molar mass of
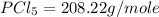
The given chemical equation is -
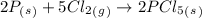 --------- 2
--------- 2
Now , calculation -
Mass of
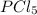 formed = 118g
formed = 118g
Molar mass of
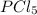 =
=
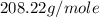
Mole =
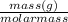
Therefore , moles of
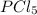 formed =
formed =
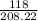
From equation 2 , we get to know that ,
2mole
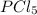 formed from 5 mole
formed from 5 mole
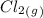
Therefore ,
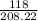 mole
mole
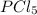 formed from
formed from
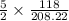 mole
mole
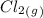
Moles of
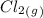 used =
used =
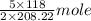
R=
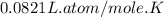
Pressure (P)= 1.85atm
Temperature (T)= 322K
Moles of
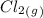 (n)=
(n)=
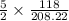 moles
moles
Applying the formula above in 1 equation , that is
PV = nRT
putting the given values -
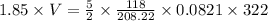
V = 20.245 litres.
Hence , the volume of the flask is 20.245 litres .