Answer:
15.64 moles
81.8% (3 s.f.)
Step-by-step explanation:
Let's start by writing a balanced equation.
N₂ +H₂ → NH₃
To balance the equation, ensure that the number of atoms of each element is the same on both side of the arrow. On the left, we have 2 N atoms and only 1 N on the right. Thus, write '2' in front of NH₃ to balance the N.
N₂ +H₂ → 2NH₃
Now, balance the number of H atoms. Currently, there are 2 Hs on the left and 6 Hs on the right. To balance the equation, write a 3 in front of H₂.
N₂ +3H₂ → 2NH₃
The equation is now balanced.
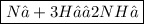
Given that hydrogen is in excess, the number of moles of NH₃ is dependent on the number of moles of N₂, which is the limiting reactant.
The mole ratio of N₂ to NH₃ produced is 1: 2.
Thus with 7.82 mol of N₂,
number of moles of NH₃
= 2(7.82)
= 15.64 moles
This is the theoretical yield since the calculations were based from the chemical equation.
However, in reality, the percentage yield may not be 100% as some products are lost in the process.
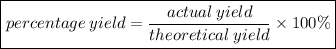
∴ Percentage yield of NH₃
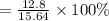
= 81.8% (3 s.f.)