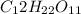Answer: 1 M
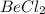 > 1 M KCl = 2 M
> 1 M KCl = 2 M
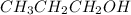 > 1 M
> 1 M
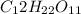
Step-by-step explanation:
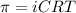
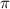 = osmotic pressure = ?
= osmotic pressure = ?
i = vant hoff factor
C= concentration in Molarity
R= solution constant
T= temperature
a) 1 M
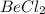
i = 3
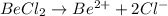
Thus Concentration of ions =
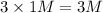
b) 1 M KCl
i = 2
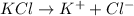
Thus Concentration of ions =
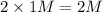
c) 2 M
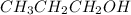
i = 1 ( as it doesnot dissociate)
Thus Concentration of ions =
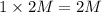
d) 1 M
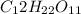
i = 1 ( as it doesnot dissociate)
Thus Concentration of ions =
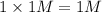
Thus order from highest to lowest osmotic pressure is:
1 M
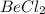 > 1 M KCl = 2 M
> 1 M KCl = 2 M
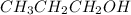 > 1 M
> 1 M