Answer:

Step-by-step explanation:
1 mole of any substance has the same number of particles: 6.022 * 10²³. This number is as Avogadro's Number.
The particles can be molecules, atoms, formula units, etc. For this problem, the particles are molecules of C₆H₁₄ or hexane.
We can set up a ratio using Avogadro's Number.
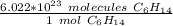
Multiply by the given number of moles: 0.655
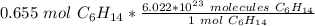
The moles of hexane will cancel, because one is the "numerator" (techincally 0.655 is over 1) and the other is in the denominator of the ratio.
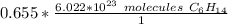
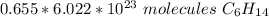
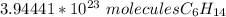
The original value of moles has three significant figures, so our answer must have the same. For the number we found, that is the hundredth place.
The 4 in the thousandth place tells us to leave the 4 in the hundredth place.
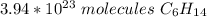
0.655 moles of hexane is equal to approximately 3.94 *10²³ molecules of hexane.