Answer:
55.1 g Si.
Step-by-step explanation:
Hello there!
In this case, for the given mole-mole relationship, it is possible to set up the following mole ratio as the moles of Si and P are the same:
1 mol Si = 1 mol P.
Next, since one mole of silicon has a mass of 28.09 g and one of phosphorous of 31.0 g, we can set up the following expression for the mass of silicon:
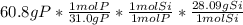
Therefore, the result is 55.1 grams of silicon.
Best regards!