Answer:
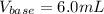
Step-by-step explanation:
Hello there!
In this case, by considering that the reaction between sodium hydroxide and hydrochloric acid is in a 1:1 mole ratio of these two reactants, we are able to use the following equation relating the concentration and volume of each one:
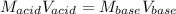
In such a way, by solving for the volume of the base, we will obtain:
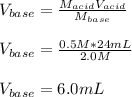
Regards!