Answer:
8.14x10⁻³ moles of N
Step-by-step explanation:
In order to solve this problem first we convert 0.179 grams of N₂O into moles, using its molar mass:
- 0.179 g ÷ 44 g/mol = 4.07x10⁻³ mol N₂O
Then we convert N₂O moles into N moles, using the data given by the compound's formula (that there are two N moles per N₂O mol):
- 4.07x10⁻³ mol N₂O *
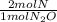 = 8.14x10⁻³ mol N
= 8.14x10⁻³ mol N