Answer:
5 mL of 5.0 M H₂SO₄ (aq) are needed to prepare 100 mL of 0.25 M H₂SO₄ (aq).
Step-by-step explanation:
In chemistry, dilution is the reduction of the concentration of a chemical in a solution.
Then, dilution consists of preparing a less concentrated solution from a more concentrated one, and it consists simply by adding more solvent to the same amount of solute. That is, the amount or mass of the solute is not changed, but the volume of the solvent varies: as more solvent is added, the concentration of the solute decreases, since the volume (and weight) of the solution increases.
A dilution is calculated by the expression:
Ci*Vi = Cf*Vf
where:
- Ci: initial concentration
- Vi: initial volume
- Cf: final concentration
- Vf: final volume
In this case, you know:
- Ci=5 M
- Vi= ?
- Cf= 0.25 M
- Vf= 100 mL
Replacing:
5 M*Vi = 0.25 M* 100 mL
Solving:
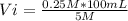
Vi= 5 mL
5 mL of 5.0 M H₂SO₄ (aq) are needed to prepare 100 mL of 0.25 M H₂SO₄ (aq).