Answer:
the concentration of the solution is 2 mol/L.
Step-by-step explanation:
Molarity is defined as the concentration of a solution expressed in terms number of moles of solute per liter of solution.
Mathematically Molarity is expressed as follow;
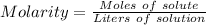
Given;
Volume of the solution, V = 0.45 L
Moles of the solute, n = 0.9 moles
The concentration of the solution
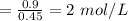
Therefore, the concentration of the solution is 2 mol/L.