Answer:
n = 0.223 mol.
Step-by-step explanation:
Hello there!
In this case, according to the given information, it is possible for us to approach this problem via the ideal gas law, which involves the pressure, moles, temperature and volume of the gas:
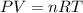
Thus, by solving for moles we obtain:
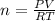
Therefore, we plug in the given volume, temperature (in Kelvins) and pressure to obtain:
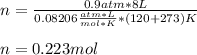
Best regards!