Answer:
10 moles of S₈
320 moles O₂
Step-by-step explanation:
The balanced equation is:
Now we can convert moles of SO₈ (the product) into moles of each reactant, using the stoichiometric coefficients of the balanced reaction:
- 80 mol SO₈ *
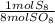 = 10 mol S₈
= 10 mol S₈
- 80 mol SO₈ *
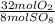 = 320 mol O₂
= 320 mol O₂
In order for 80 moles of SO₈ to be produced, 10 moles of S₈ need to react with 320 moles of O₂.